The bonds that unite molecules tell the most fascinating stories in the complex field of biochemistry, since chemistry is the language of life. These bonds—which can be strong or weak—are essential in forming the molecules that carry out biological functions.
This investigation explores the various kinds of chemical bonds that are present in biochemistry, revealing their properties and the energy, expressed in kilocalories (kcal), that they hold.
 |
| Attribution: Aherthabey, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons |
Covalent Bond
The strong foundation of biomolecules such as proteins, carbohydrates, lipids, and nucleic acids is provided by covalent bonding. Strong connections are formed when atoms share electron pairs, forming these bonds. Covalent connections can be broken to release energy, which powers essential biological functions. A covalent bond can be a single, double, or triple bond.
A polar covalent bond is a type of chemical bond that occurs when two atoms share a pair of electrons unevenly. This happens when one atom has a higher electronegativity than the other, leading to an uneven distribution of electrons within the bond. As a result, the atoms involved in the bond develop partial positive and negative charges.
Equal electron sharing between two atoms produces a non-polar covalent bond, which has a balanced charge distribution and no permanent dipole moment.
 |
| Attribution: MikeRun, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons |
Disulfide Bond
 |
| Attribution: Berichard, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons |
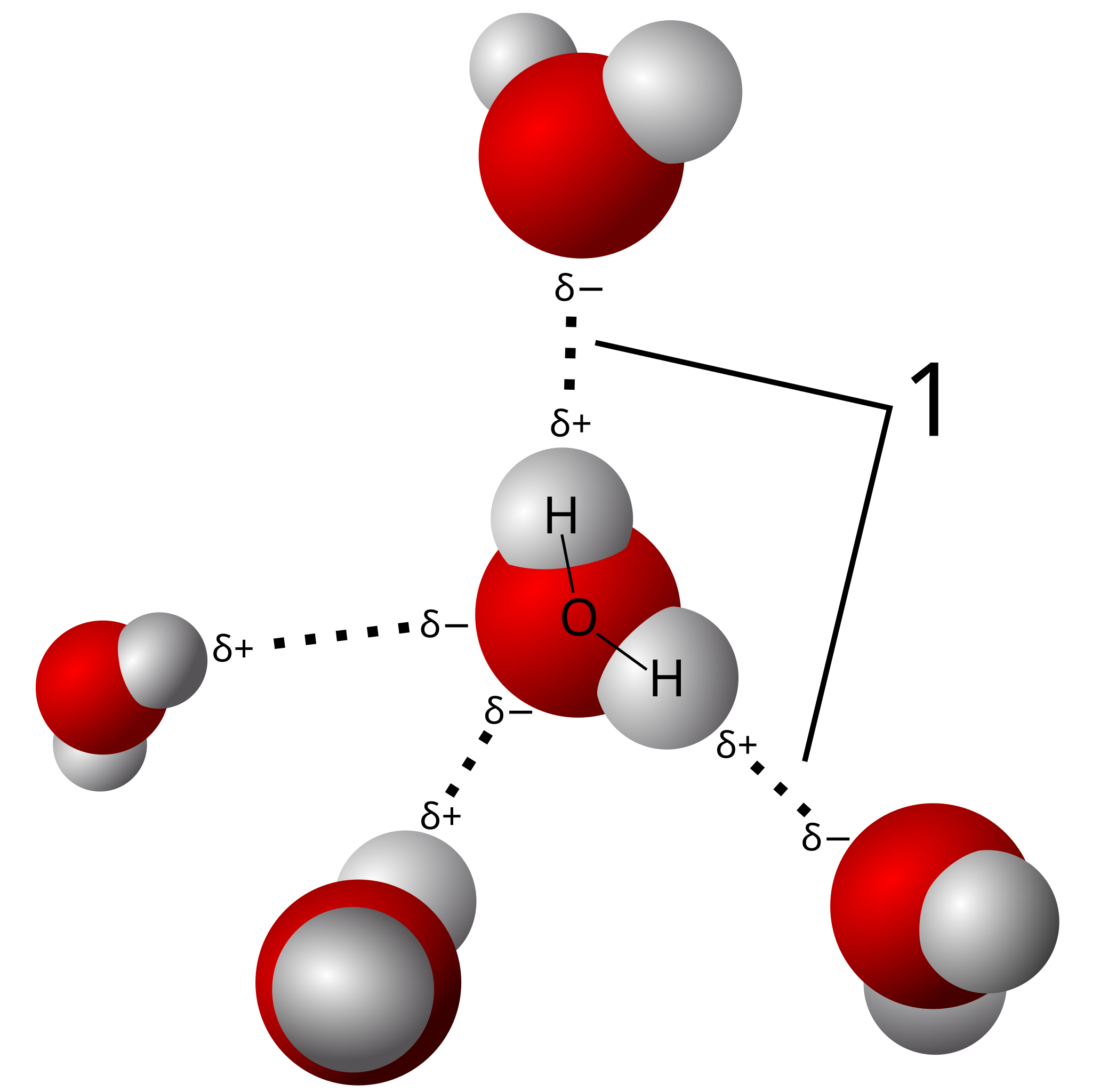
Hydrogen Bond
Even though hydrogen bonds are weak on their own when combined, they act as biomolecules' molecular Velcro, binding complex structures together. In polar covalent bonds, these bonds are formed between hydrogen atoms and electronegative atoms. Their comparatively low energy is essential for preserving biomolecular structures since they aid in the stability of protein structures and base pairing in nucleic acids.
 | ||||||||||
Ionic Interaction
The attraction between positively and negatively charged ions is the source of ionic interactions. They aid in the ligand-receptor binding process and the stability of protein structures. The amount of charge and the separation between the ions affect how much energy is used in these interactions. Ionic interactions are a dynamic aspect of biochemistry that regulates cellular processes and protein activity.
 |
| Attribution: Wdcf, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons |
Van der Waals Forces
In biomolecules, van der Waals forces—such as dipole-dipole interactions and London dispersion forces—provide subtle attraction. The variations in the distribution of electrons within molecules give rise to these weak, short-range forces. The aggregate energy of van der Waals interactions contributes to the stability of biomolecular complexes by enabling protein-ligand binding and protein-protein interactions, which help molecules stick to one another.
The links between biomolecules are the choreographers of the complicated dance that is life. Every link in biomolecules, from the strong covalent bonds that support them to the minuscule hydrogen bonds that form their complex structures, plays a vital role in the robustness and efficiency of biological systems. Not only may this knowledge help to explain the workings of life's machinery, but it also provides new opportunities for the development of therapies and interventions that specifically target the molecular interactions involved in biochemistry.

.png)
