Chemistry, which is frequently referred to as the "core science," is based on a deep comprehension of the makeup of molecules, the structure of atoms, and the properties of chemical bonding. These ideas form the basis of chemistry and offer a framework for understanding the behaviour and interactions of matter at the most basic level. We will go on an insightful exploration of the structures, characteristics, and roles that atoms, molecules, and chemical bonds play in determining the physical and chemical properties of substances in this informative blog.
 |
| Attribution: Richie Bendall, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons |
1.1 Atomic Structure:
The fundamental building blocks of matter, atoms are made up of three primary subatomic particles: protons, neutrons, and electrons. The nucleus's dense core is made comprised of protons and neutrons, while electrons orbit the nucleus at different energy levels, called shells. Since protons are positively charged, the atom's charge is neutralized by the negatively charged electrons that surround the nucleus.Ninety six percent of living matter consists of C, H,O, and N. 3% is P, Ca, S, and K.
Isotopes are like siblings from the same family—they belong to the same element, have the same number of protons , but differ in neutrons, which changes their weight.e.g., Carbon-12 (₆C¹²) and Carbon-14 (₆C¹⁴) Isobars are like strangers with the same birthday (mass number) but different names (elements), e.g.Argon-40 (₁₈Ar⁴⁰) and Calcium-40 (₂₀Ca⁴⁰). Isotones are atoms that don’t share the same element, but have the same number of neutrons—like having the same mood but different personalities, e.g. Carbon-14 (₆C¹⁴) and Nitrogen-15 (₇N¹⁵).
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Some isotopes are stable, while others are unstable or radioactive. These radioactive isotopes decay over time, releasing energy and particles in a process known as radioactive decay.
This decay is nature’s way of restoring balance in an unstable nucleus. It doesn't happen all at once but follows a predictable pattern, governed by something called a half-life—the time it takes for half of the isotope’s atoms to decay.
Types of Decay:
- Alpha Decay (α) – The nucleus releases 2 protons and 2 neutrons (like a helium atom). This usually happens in heavy elements like uranium or radium.
- Beta Decay (β) – A neutron turns into a proton (or vice versa), and an electron (or positron) is emitted. This changes the identity of the element.
- Gamma Decay (γ) – The nucleus loses excess energy in the form of gamma rays, without changing the number of protons or neutrons.
Real-life Relevance:
- Carbon-14 dating uses the decay of carbon isotopes to estimate the age of ancient artifacts.
- Medical scans use short-lived isotopes like Technetium-99m to visualize organs.
- Nuclear energy relies on isotope decay for heat generation.
In simple words: decay is how an unstable atom transforms into something more stable, while releasing energy that can either help us, harm us, or reveal secrets of the past.
 |
| Credit: AhmadSherif, Public domain, via Wikimedia Commons |
1.2 Electron Cloud Model:
The likelihood of discovering electrons in particular areas surrounding the nucleus is described by the electron cloud model, which is based on quantum mechanics. These areas, which are frequently represented as electron shells or orbitals, correlate to energy levels where electrons are most likely to be present. A certain amount of electrons may be accommodated by each shell, with higher energy levels able to contain more electrons than lower ones.
 |
| Attribution: Geek3, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons |
1.3 Atomic Number and Mass Number:
Every element in the universe has its own identity, and at the heart of that identity lie two simple numbers: the atomic number and the mass number. The atomic number tells us how many protons are in an atom's nucleus — it’s like the fingerprint of an element. No two elements share the same atomic number, which is why it’s so important in chemistry. For example, if an atom has 6 protons, it’s always carbon. Then there’s the mass number, which adds up the protons and neutrons to give us an idea of how heavy the atom is. While protons and neutrons sit packed in the nucleus giving the atom its mass, tiny electrons just float around and are too light to count in. These two numbers might seem small, but they help us understand how atoms behave, how they react, and even why some atoms are more stable than others. It’s a simple yet powerful way of decoding the language of matter. The mass of a proton is approximately 1.6726 × 10⁻²⁷ kilograms, and it carries a positive charge of +1.602 × 10⁻¹⁹ coulombs. The neutron has a mass of about 1.6749 × 10⁻²⁷ kilograms but carries no electric charge. In contrast, the electron is much lighter, with a mass of around 9.1094 × 10⁻³¹ kilograms, and it has a negative charge of −1.602 × 10⁻¹⁹ coulombs.
Part 2: Molecules - The Architectural Marvels of Chemistry
2.2 Types of Molecules:
Based on their composition and bonding characteristics, molecules can be classified into several different categories. Elemental atoms bound together to form simple molecules, such as oxygen (O₃) and hydrogen (H₃). Different types of atoms are bound together to form compound molecules, such as carbon dioxide (CO₂) and water (H₂O). The building blocks of biochemistry are carbon-based organic compounds, which are the basis of life on Earth.
Part 3: Chemical Bonds - The Forces that Bind Atoms Together
3.1 Covalent Bonds:
To reach a stable electron configuration, atoms share electrons to form covalent bonds. These bonds are defined by the sharing of electron pairs between atoms, which forms unique molecules with unique structures and characteristics. Depending on the difference in atoms' electronegativity, covalent bonds can be either polar or nonpolar.
 |
| Attribution: MikeRun, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons |
3.2 Ionic Bonds:
When atoms exchange electrons to form a stable ion configuration, ionic bonds are formed. Positively charged cations and negatively charged anions are formed as a result of this transfer, and they are drawn to one another by electrostatic forces. Strong ionic interactions cause ionic compounds to have high melting and boiling temperatures, such as potassium iodide (KI) and sodium chloride (NaCl).
 |
3.3 Hydrogen Bonds:
A hydrogen atom is bound to an electronegative atom (such as nitrogen or oxygen) and another electronegative atom forms weak electrostatic bonds known as hydrogen bonds. Despite their inherent weakness, hydrogen bonds are essential for maintaining the structural integrity of complex structures such as proteins and nucleic acids, and for modifying the characteristics of substances like water.
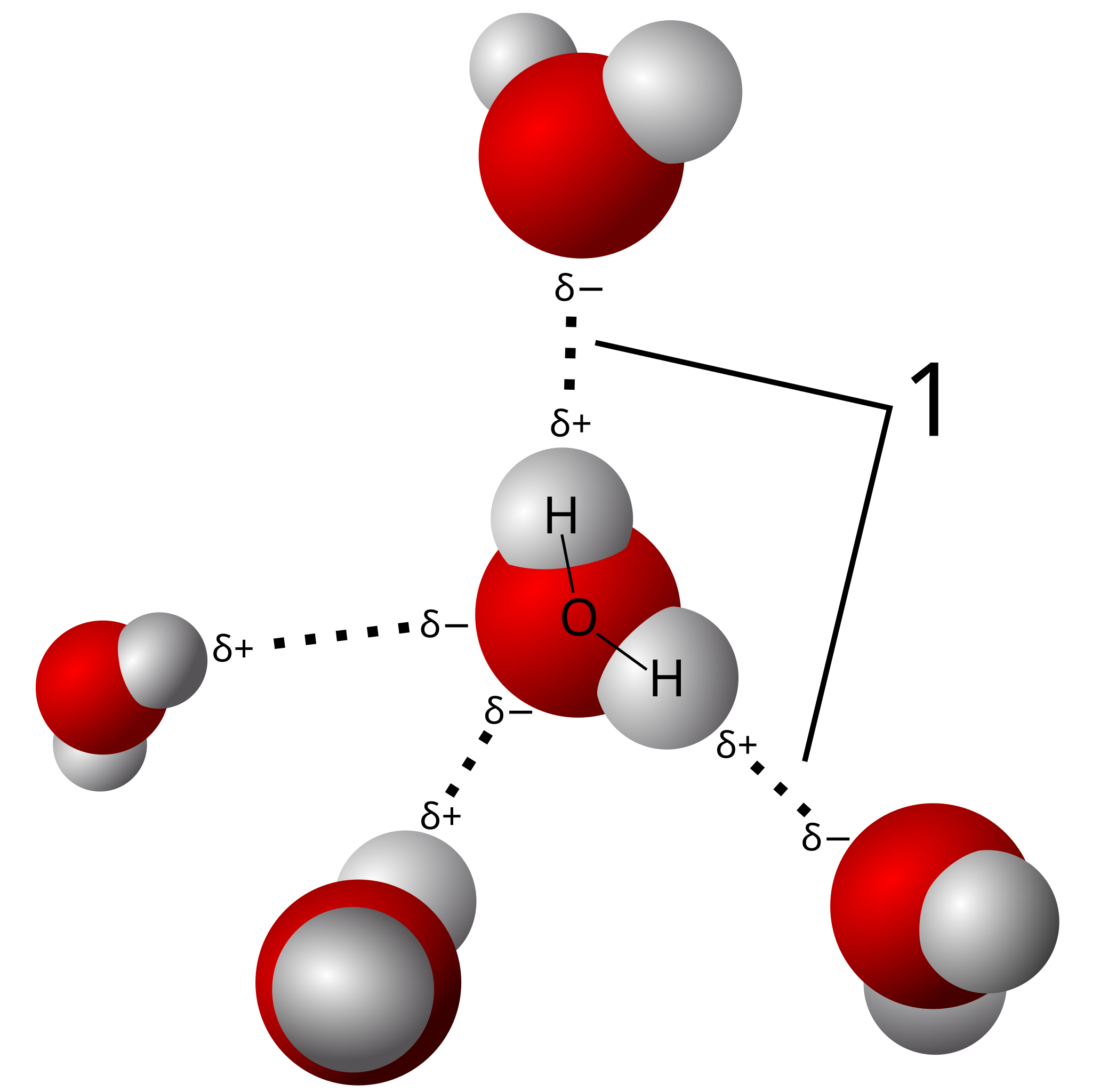 |
Conclusion:
Chemistry as a whole is based on the structure of atoms, molecules, and chemical bonds, which offer a framework for comprehending the makeup, characteristics, and changes of matter. Our understanding of the constitution of the physical universe and the principles underlying chemical reactions is greatly enhanced by exploring the complexities of atomic structure, molecule makeup, and bonding forces. Our growing comprehension of the cosmos and advances in science and technology is made possible by the beauty and complexity that are inherent in the basic elements of the universe, which we unearth through ongoing inquiry and discovery.
